J. J Thomson's father died when he was only sixteen years old. He studied in Owens College, Manchester. He was encouraged by his professor to take up a scholarship in Trinity College. He topped second in his class in the year 1880 next to Joseph Larman. Trinity gave him a fellowship and he stayed while trying to craft models to reveal the nature of atoms and electromagnetic forces. In the year 1884, He was recruited in the Cavendish Laboratory. He was the third Cavendish professor there. He was one of the most accomplished physicists of his era. In the year 1906, He was awarded the Nobel Prize in Physics for his researches in the discharge of electricity in gases. In 1918, he was chosen the master of his old college, Trinity. A year later, he resigned from his professorship in Cavendish.
Category: British physicist and Nobel laureate
Date of birth: December 18, 1856
Date of death: August 30, 1940
Wife: Miss Rose Paget
Wife: Miss Rose Paget
Profession: Physicist, Mathematician
Works written: Electricity and matter, ...
Awards and recognition
Royal Medal (1894)
Hughes Medal (1902)
Nobel Prize for Physics (1906)
Elliott Cresson Medal (1910)
Copley Medal (1914)
J. J Thomson was one of the great scientists of the 19th century; his inspired and innovative cathode ray experiment greatly contributed to our understanding of the modern world.
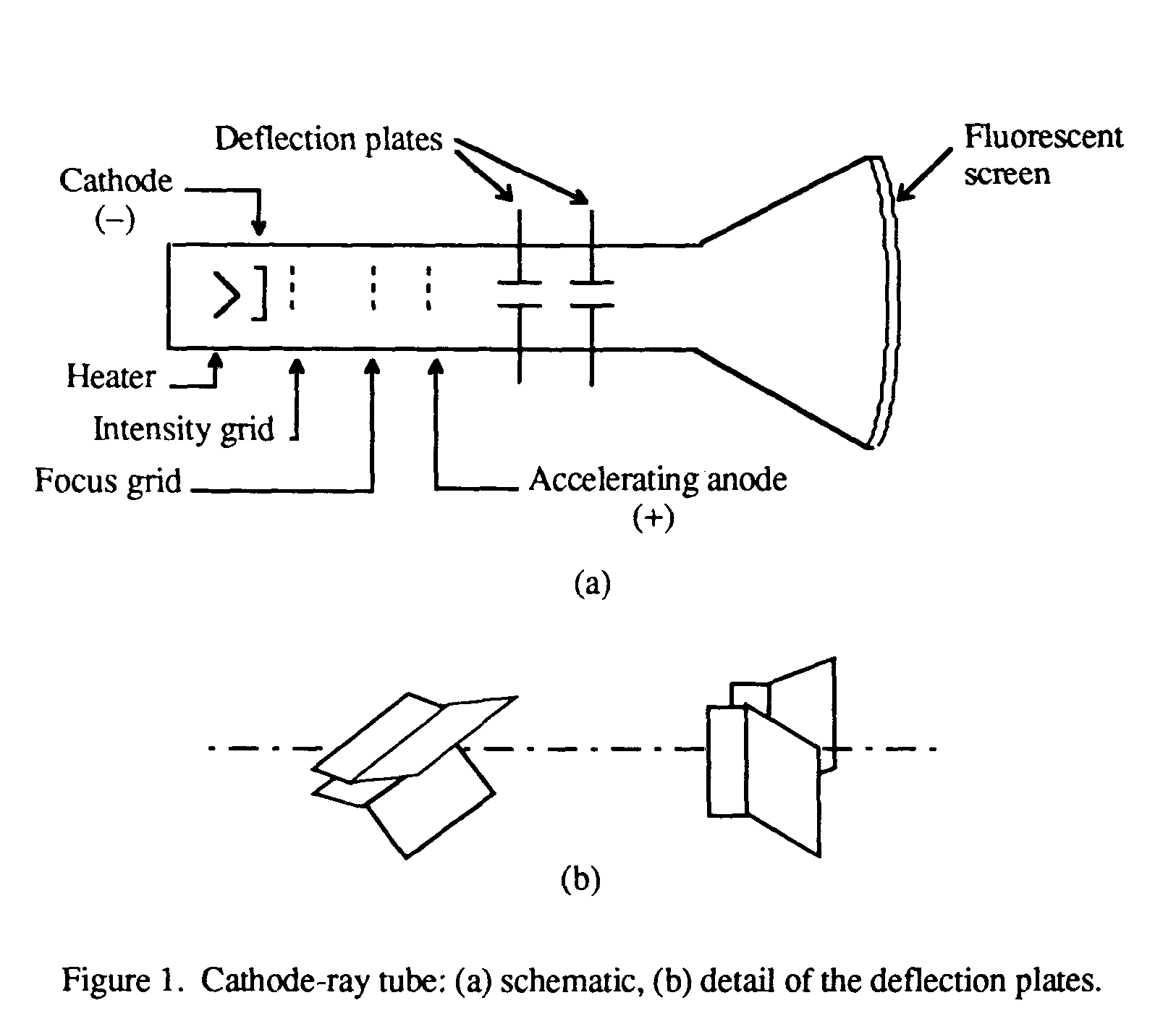
CATHODE RAY TUBE
it is a glass tube with wires inserted at both ends. It pumps out as much of the air as they couds and the electric charge passed across the tube would create a flourescent glow. Its commonly seen as the glowing neon sign on any "old fashioned" television.
JJ THOMSONS CAHTODE RAY: SECOND EXPERIMENT
CATHODE RAY TUBE
it is a glass tube with wires inserted at both ends. It pumps out as much of the air as they couds and the electric charge passed across the tube would create a flourescent glow. Its commonly seen as the glowing neon sign on any "old fashioned" television.
JJ THOMSONS CAHTODE RAY: SECOND EXPERIMENT
He had to construct a slightly different cathode ray tube. He attempted to deflect the rays with an electric field. Later on, he proved that the rays were delfected by the electric charge. Thus, it carried a negative charge.
JJ THOMSONS CATHODE RAY: THIRD EXPERIMENT
Thomson found out the charge ratio was so high that the particles either carried a huge charge or a thousand times smaller than a hydrogen ion.
To know more about J.J Thomson and his works, visit


No comments:
Post a Comment